A bioprocessing chromatography skid, often referred to as a chromatography system or unit, is a critical component in biopharmaceutical manufacturing. These systems are used to purify and separate biotherapeutic molecules, such as proteins, antibodies, vaccines and cells, from complex mixtures. The high degree of automation allows for the precise control and monitoring of the purification process ensuring consistency and process efficiency. This results in higher purity levels, yield, and product consistency while adhering to regulatory standards.
Verdot specializes in process Chromatography skids from lab-scale through clinical trials and commercial large-scale manufacturing. We understand that the needs and requirements for each process workflow may be unique. Our portfolio encompasses both standard and custom stainless steel multi-use and single-use chromatography skids depending on your biologic purification application. Refined manufacturing processes and comprehensive quality system ensure that we provide superior-quality products to our customers.
Contact us for more information.
Process Chromatography Skids – 01 to 05
 Customizable stainless steel multi-use chromatpgraphy skids capable of flow rates up to 5,000 L/hr.
Customizable stainless steel multi-use chromatpgraphy skids capable of flow rates up to 5,000 L/hr.
FlexiPro™ Single-use Process Chromatography System
 Single-use benchtop system specifically designed for small- to pilot-scale GMP bioprcessing workflows. Choose from four disposable flow kits option giving the largest flow rate on the market (0.6 – 600 L/hr.)
Single-use benchtop system specifically designed for small- to pilot-scale GMP bioprcessing workflows. Choose from four disposable flow kits option giving the largest flow rate on the market (0.6 – 600 L/hr.)
MiniPro™ Benchtop Chromatography System
Versatile process chromatography benchtop system for small-scale GMP bioprcessing workflows. Configurable two pump-head configuration supporting 4-120 L/hr. flow rates
Custom Systems for Downstream Bioprocessing
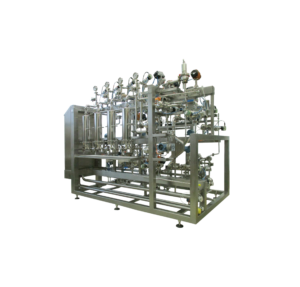
With over 35 years of building custom products in the bioprocessing space, we have the experience and scientific understanding to effectively consult, design, manufacture and validate a system based on your needs and process requirements. Our refined manufacturing processes and comprehensive quality system ensure that we deliver superior-quality products. Systems can be designed to meet various regulated requirements along with 21CFR part 11 compliance data integrity standards.
Ready to take your innovation to market?
Reach out to learn more about how we can help you with your downstream processing needs.


